If you need to register a medical device, we are here to help you.
Russian medical device market is the 5th largest in the world, and continues to grow.
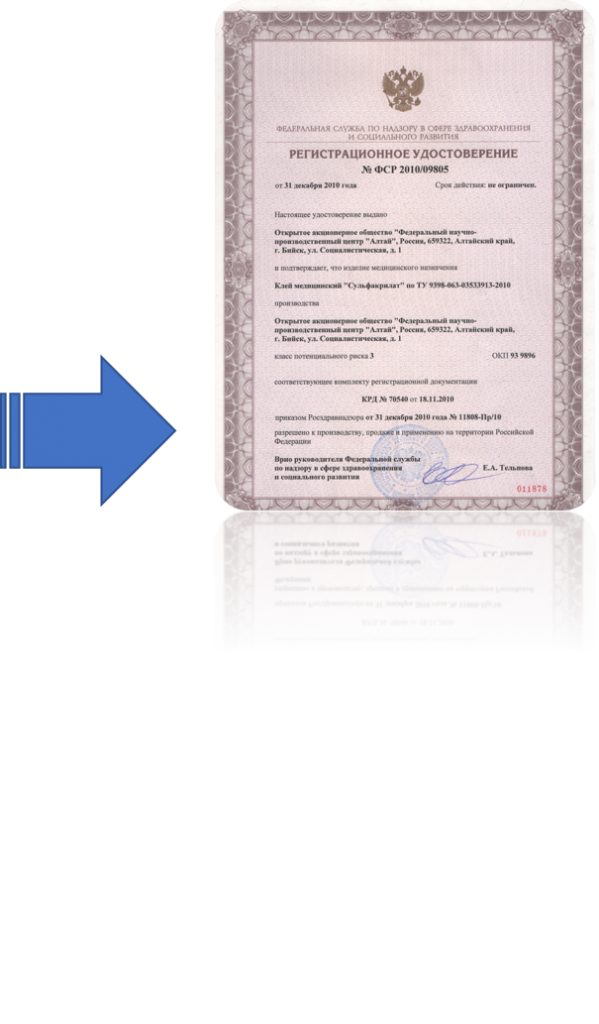
Russia is also the largest consumer base in Europe, with over 55 million households.In order to gain access to Russian market and place a medical device on it, the manufacturer must first obtain the Registration Certificate of Roszdravnadzor (Federal Service for Surveillance in Healthcare).Registration of medical device is a complicated state procedure, which includes application, registration dossier preparation according to Russian legislation, functional, toxicological (biocompatibility), EMC and metrology tests (if applicable), documentation review and expertise by Roszdravandzor, clinical trials and state expertise.Elmas provides full support of this procedure, from getting the necessary documents from the manufacturer to receiving the issued Registration certificate from Roszrdavnadzor. We also supply you with full range of additional services — should you require them: starting with Authorized Representative to translations to notary legalization.
Definition of a medical product according to Russian regulatory system
«Any instrument, device or material or other product used for medicinal purposes alone or in combination with another medical devices designed for:
Prevention, diagnosis, treatment, rehabilitation of diseases
Restoration, replacement of anatomical structures or physiological functions of the body
Monitoring condition of the human body
Medical research
and the functional purpose of which is not achieved through pharmacological, immunological, genetic or metabolic effects on the human body.»
![]()
Risk based classification according to Russian regulatory system
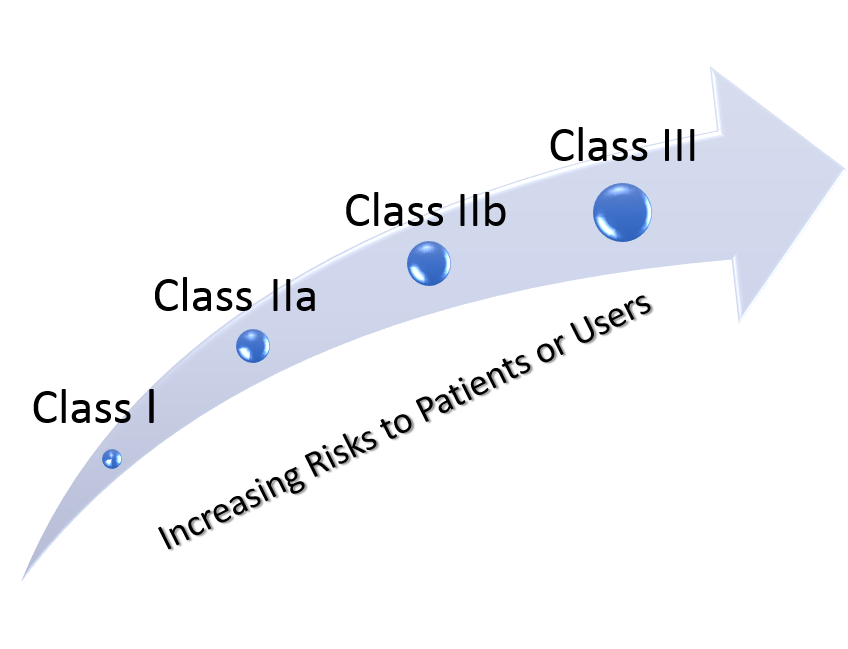
According to Russian regulatory system, all medical products and devices are classified in accordance with their degree of potential risk to patients and users and fit into four classes: Class I, IIa, IIb and III.
The degree of potential risk from usage of medical product increases respectively with the class.
Each medical product or device belongs only to one class, similar to European classification criteria for medical products according to Annex IX of MDD 93/42/EEC
Registration workflow
Registration dossier preparation and product sample testing
Risk class: I Risk class: IIa, IIb, III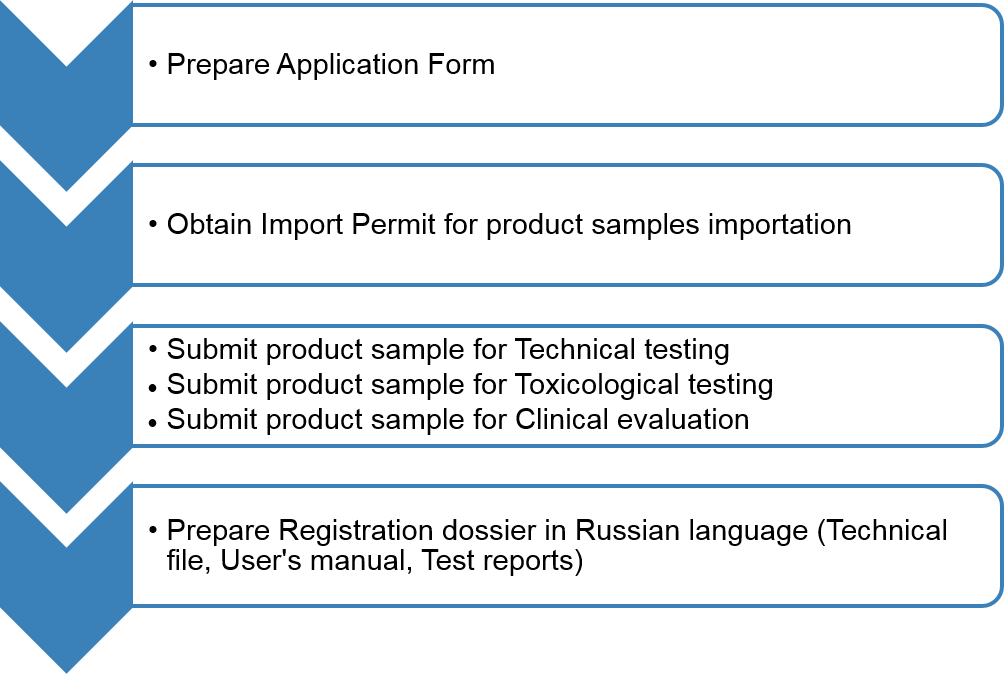

Documents checklist
Depending on product type, additional documents may be required for submission as well
- Manufacturer's business registration
- Medical product specifications
- User Manual
- CE certificate (if available)
- ISO 13485
- Declaration of Conformity (for I, IIa risk-class products)
- Biocompatilibility test report
- Risk management file
- Medical product manufacturing flowchart
- Clinical evaluation (clinical data)
- Available engineering/laboratory test reports (Safety/EMC)
Registration dossier submission to Roszdravnadzor
Risk class: I Risk class: IIa, IIb, III

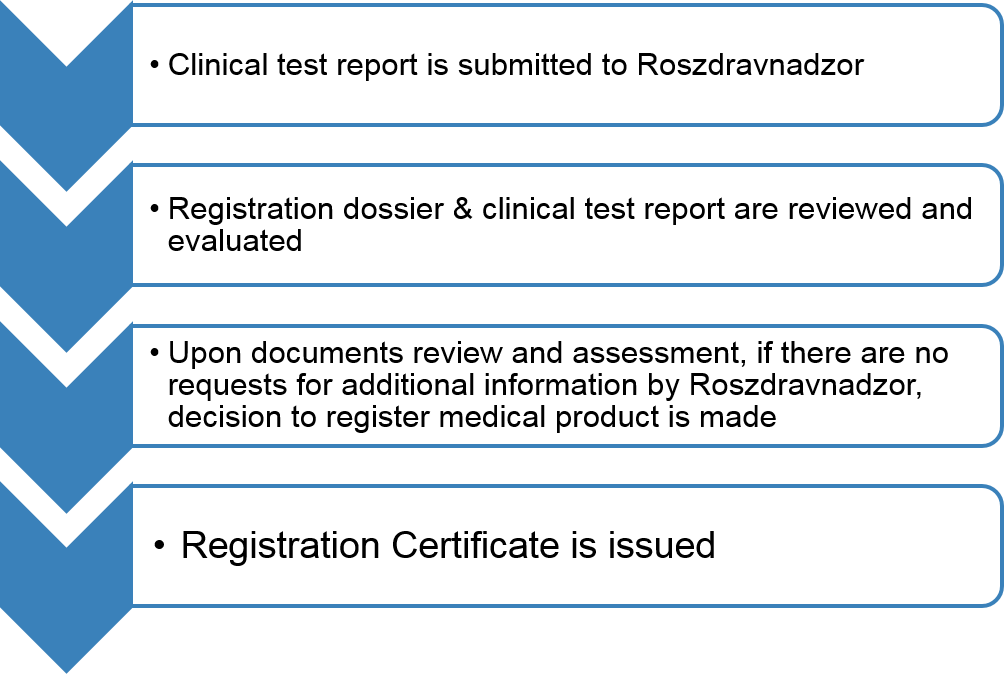
Clinical test report is submitted to Roszdravnadzor for evaluation